Clinical Research
Carpal tunnel release using ultrasound (US) guidance was initially described by Nakamichi in 1997.12,13
Since then over 1200 cases have been reported in the peer-reviewed literature using a variety of cutting knives or instruments not specifically designed for CTR using US Guidance.14,15
Despite these limitations, there have been no reported neurovascular complications and the collective clinical success rate has been over 95%.14,15
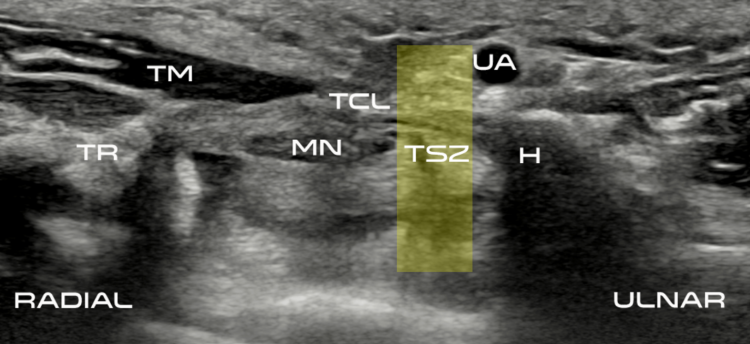
Clinical Experience
The first CTR using the SX-One MicroKnife® with US guidance was performed on February 17, 2017.
- Over 7700 procedures completed, including many simultaneous bilateralreleases.15-17
- Over 60 physician users in 23 different states.
- Procedures performed in the ASC, OR and clinic office setting – most procedures performed using only local anesthesia/WALANT technique.15-18
- Post-operative discomfort typically managed with acetaminophen or NSAIDs as necessary: no opioids required.15-18
- Patients are generally allowed to resume activities as tolerated (at physician’s discretion).
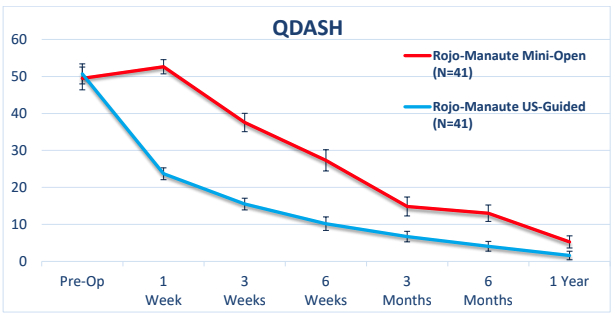
CTR using ultrasound guidance V.S. mini-Open CTR (Randomized Clinical Trial)
Data reproduced from a prospective, randomized trial comparing the two techniques (mini-open CTR without US guidance (red line) and CTR with US guidance (blue line) showed that patients treated with CTR using US guidance recovered significantly faster during the first 3-6 weeks compared to traditional mOCTR.9
“Purpose: The purpose of this study was to report the 1-year clinical outcomes of carpal tunnel release using ultrasound guidance (CTR-US)performed in a large, real-world population of patients enrolled in a multicenter registry.”
Click ‘Click Here’ to read the study.

